-
Degrees
- Program Finder
-
Bachelor's Degrees
- Accounting
- Analytics
- Applied Management
- Business Administration
- Business Forensics
- Cloud Computing
- Communications
- Computer Science
- Criminal Justice Administration
- Cybersecurity
- Education
- Exercise Science
- Financial Management
- Financial Planning
- Forensic Accounting
- Healthcare Administration
- Human Resources Management
- Information Systems
- Information Technology
- Management & Leadership
- Management Science
- Marketing
- Nursing (RN-BSN)
- Operations & Supply Chain Management
- Psychology
- Public Health
- Public Safety Management & Leadership
- Social Sciences
- Sport Management
- User Experience & Graphic Design
-
Master's Degrees
- Accounting
- Business Analytics
- Business Psychology
- Communications
- Computer Science
- Criminal Justice
- Cybersecurity
- Data Analytics
-
Education
- M.Ed. in Educational Leadership-Higher Education Leadership
- M.Ed. in Educational Leadership-K-12 Building Level Leadership
- M.Ed. in Educational Leadership-Principal Licensure
- M.Ed. in Instructional Design & Technology-Curriculum & Instruction in Higher Education Specialization
- M.Ed. in Instructional Design & Technology-Curriculum & Instruction in K-12 Education Specialization
- M.Ed. in Instructional Design & Technology-Training & Development Specialization
- Exercise Science
- Health Informatics
- Healthcare Administration (MHA)
- Human Resource Management
- Information Systems
- Information Technology
- MBA Programs
- MSN Programs
- Professional Sales Leadership
- Psychology
- Public Administration (MPA)
-
Doctoral Degrees
- Associate Degrees
-
Online Degrees
- Online Learning at Franklin
- Accounting Programs
- Business & Leadership Programs
- Computer Science Programs
- Criminal Justice & Public Safety Programs
- Cybersecurity Programs
- Data & Analytics Programs
- Education Programs
- Finance Programs
- Healthcare Programs
- Human Resources Programs
- Information Technology Programs
- STEM Programs
- Marketing & Communications Programs
- Nursing Programs
- Operations & Project Management Programs
- Psychology Programs
- Public & Social Sciences Programs
- Online Learning Facts
- Degrees By Location
- Degrees By College
- Minors
- Bachelor’s & Master’s Combined Programs
- Degree Completion Programs
- Second Bachelor's Degrees
- STEM Degrees
-
Microcredentials & Certificates
- Microcredentials
-
- Accounting Data Analytics
-
- Cyber Defense
- Cyber Governance
- Data Analytics
- Nurse Educator
-
- Adult-Gerontology Primary Care Nurse Practitioner
- Family Nurse Practitioner
- Psychiatric Mental Health Nurse Practitioner
- Ohio Superintendent License
-
- Adolescence to Young Adult Education (7-12)
- Primary Education (PK-5)
- Intervention Specialist: Mild-Moderate
- Intervention Specialist: Moderate-Intensive
- Middle Childhood Education (4-9)
-
Admissions
- Undergraduate Students
- Master's Students
- Doctoral Students
- Partnership Students
-
- Study in the U.S.
- Earn Your Degree Online
- Community College Students
- College Credit Plus
-
-
- Air Force
- Army
- Coast Guard
- Marines
- Navy
-
- Montgomery GI Bill® - Selected Reserves
- Montgomery GI Bill®
- Post-9/11 GI Bill®
- Vocational Rehabilitation and Employment VetSuccess Program
- Yellow Ribbon Program
- Spouses & Family
-
- Online Open House
-
- Associate Degrees
-
- General Bachelor's Degree
- Nursing (RN-BSN)
-
- Accounting
- Business Administration (MBA)
- Business Analytics
- Business Psychology
- Computer Science
- Criminal Justice Administration
- Cybersecurity
- Data Analytics
- Health Informatics
- Healthcare Administration (MHA)
- Human Resource Management
- Information Technology
- Instructional Design & Learning Technology
- Nurse Administrator (MSN-ADM)
- Nursing-Adult-Gerontology Primary Care Nurse Practitioner
- Nursing-Family Nurse Practitioner (MSN-FNP)
- Nursing-Generalist (MSN)
- Nursing-Psychiatric Mental Health Nurse Practitioner
- Public Administration
-
- Business Administration (DBA)
- Healthcare Administration (DHA)
- Instructional Design Leadership (DPS)
- Nursing Practice-Family Nurse Practitioner (DNP-FNP)
- Nursing Practice-Leadership Track (DNP)
- Organizational Leadership (Ed.D.)
-
- Criminal Justice Leadership
-
Tuition & Financial Aid
- Tuition & Fees
- Cost Estimate Calculator Tool
- Tuition Guarantee
- Undergrad Tuition Comparison
- Federal Aid & State Aid
- Institutional Aid & Private Loans
- Applying for Aid
- Community College Students
- Scholarships
- Payment Options
- Financial Aid Resources
-
Transferring Credit
- Estimate Your Transfer Credit
- How to Transfer Credits
-
- Previously Earned College Credit
- Certificates + Professional Training Credit
- Military Training Credit
- Testing Credit
-
- Partner Schools
- Pathway Portal
- Transfer into a Bachelor’s Degree
- Transfer into a Master’s Degree
- Transfer into a Doctoral Degree
- Transfer into an Associate Degree
-
The Franklin Experience
- Built For Working Adults
- Transfer Friendly
- Accredited University & Quality Degrees
- Fast & Affordable
- Student Success Stories
- Valued By Employers
- Nonprofit
- Student Support
- Career Development
- Doctoral Journey
-
About Us
-
-
- Columbus
-
-
-
- Become a Partner
- Current Partners
- Teachers
-
-
- Benefits for Community Colleges
- Benefits for Businesses
-
- Employer Partnerships
-
- Solutions
-
- Al Baha University
- MCBS
- Saudi Electronic University
- Current Partnerships
- Medical School Partnerships
- Nurse Preceptor
- Ohio School District Partnerships
-
-
- Board of Trustees
- University Leadership
- University Directory
- Faculty Profiles
- President's Welcome
- Admission Advisors
-
-
- CCNE Accreditation
- IACBE Accreditation
- State Authorization & Professional Licensure Information
- Our Mission & Values
- The Four Cornerstones
- Our History
- Consumer Information
- Safety & Security
- Bookstore
- Assessment
- Map & Directions
-
-
-
- Students
- Faculty & Staff
- Future Students
- Events
- News
- Request an Expert
- Presentations & Awards
- Faculty Awards and Recognitions
- Speakers' Bureau
-
-
- Title IX
- Discrimination Harassment & Sexual Misconduct
- Anti-Hazing Policy
- Drug Free School & Communities Act
- Franklin Intervention & Awareness Team
- Filing Complaints
-
- Research at Franklin University
- Programs & Support
- Resources
- Research Opportunities
- About Us
- Office of Accessibility Services
- Combating Copyright Infringement
- Financial Aid Statement
- Influenza Information
- Information Technology Acceptable Use
- Notice of Privacy Rights (FERPA)
- Privacy Statement
- Student Parking
- Tuition Refund Policy
- Vaccinations
- Inclement Weather Policy
- Transfer Credit Policy & Procedures
- Community Engagement
- Request Your Franklin Transcripts
- Urbana University Resources
- Give to Franklin
-
Cayuse
Franklin University uses Cayuse, a cloud-based software, for IRB protocol management. Investigators use smart forms in Cayuse to complete their IRB applications and any subsequent submissions to the IRB, such as modifications to approved studies and study closures. All project documents are kept in a centralized location that is accessible to each listed investigator on a protocol.
Please review the content below for guidance on how to open a study. There are FAQs at the bottom of this page that address the most common questions we receive from users.
Opening a Study
To open a study in Cayuse, please follow the steps below. Alternatively, you may click here for a more detailed description (with screenshots).
- Franklin University uses single sign-on (SSO) to access Cayuse. Go to https://franklin.app.cayuse.com and log in using your Franklin credentials. After logging in, you will see your ‘Home’ page.
- On your home page, go to the top of your screen, on the right, and click on ‘Products’. You will have two choices: ‘Home’ and ‘Human Ethics’. Click on ‘Human Ethics’ and you’ll arrive at your dashboard.
- If you are opening a study in Cayuse, look for the blue ‘+ New Study’ tab in the upper right corner of your dashboard. Click on the new study tab.
- After you have clicked on the new study tab, you will see a screen that asks you to enter the title of your project. Once you enter a title, click on the checkmark.
- After the title of your study has been entered, you are now ready to open a submission. Click on the blue ‘+ New Submission’ tab.
- Your only choice will be to select 'Initial'. This initial submission is your IRB application.
- On the initial submission page that opens, click on the 'Edit' tab on your left.
- After you click on the 'Edit' tab, you will see the first section of your IRB application.
Any additional submissions you open in Cayuse, such as a 'Modification' or 'Closure', are started the same way. You will open your study in Cayuse and click on the blue ‘+ New Submission’ tab. Once your initial submission has been approved by the IRB, clicking on the new submission tab will present additional submission choices (Modification, Incident, or Closure).
FAQs
You'll first need to access your submission. From your dashboard, look at the center of your screen and you will find your study under 'My Tasks'. Click on your study to complete the submission.

Click on 'Complete Submission' under 'Required Tasks'.

You will see your application. At the bottom of the blue 'Sections' column on the left side of your screen, you will find the 'Complete Submission' button. Click on the button.

Confirm that you want to continue with completing your submission.

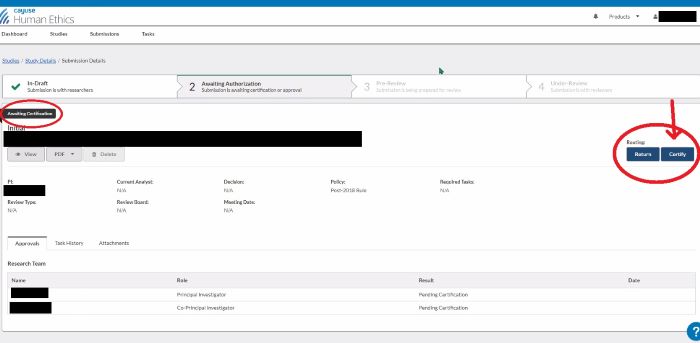
After submitting your application, you still need to certify it. When you see this screen after confirming your submission, click on the blue 'Certify' button, under 'Routing', on the right side of your screen:

If you need to reopen your submission for any reason, you have the option to 'Return' the submission prior to certification.
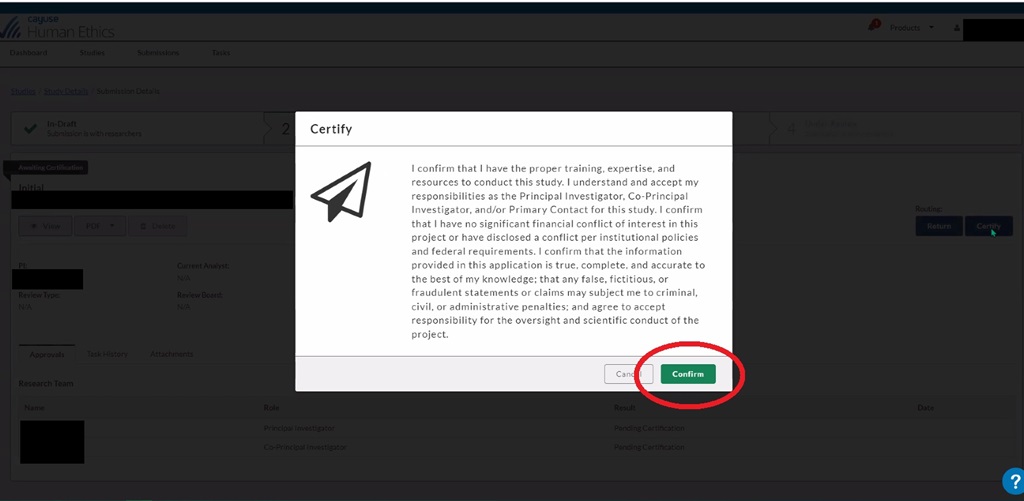
Click 'Confirm' to certify your submission.

If you are a doctoral candidate, your certified submission will route to your dissertation chair for review and certification. You can see in Cayuse when the submission has been certified by the dissertation chair.

You can check the status of your submission in Cayuse. From your dashboard, you can click on 'Awaiting Authorization' to see if your dissertation chair has reviewed and certified the submission.

Once all relevant investigators have reviewed and certified the submission, it will route to the IRB Office for review.
If you cannot submit your application because the submit button is nowhere to be found, check the blue sections column on the left side of your application screen. When all required information has been provided in a section, that section will have a checkmark next to it. If a required piece of information is missing, there will be no checkmark.
If you look at the screenshot below, you can see there is no checkmark next to 'Research Determination'. This means that required information is missing from that section.

If you click on the section that does not have a checkmark, carefully scroll through the section to see what is missing. In this case, the committee approval attachment is missing:

Missing attachments and not adding the Principal Investigator (see 'Personnel') are the most common reasons we see for a missing checkmark.
Not certifying your submission may delay the IRB's review of your application. This is because certifying a submission is part of the routing process, and an uncertified IRB submission will not automatically route to the IRB Office. Instead, the submission will sit in 'Awaiting Authorization' until it is manually moved to 'Pre-Review.'
To certify your submission, you need to open your study and go to the submission. You will see this screen after opening your submission and/or after confirming your submission. Click on the blue 'Certify' button, under 'Routing', on the right side of your screen.

Click 'Confirm' to certify your submission.

Once all relevant investigators have reviewed and certified the submission, it will route to the IRB Office for review.
Once required investigators have reviewed and certified the submission, it will route to the IRB Office for review. This initial review is called pre-review and is conducted by IRB staff. In most cases, staff read through the entire submission very carefully, leaving notes, comments, and questions for the investigator. Sometimes, however, if the investigator is missing key information or requirements (e.g., a lack of committee approval to proceed to the IRB, an inadequate research proposal, and so on), staff return the submission without a thorough review and list the reasoning behind doing so.
IRB staff have several responsibilities during pre-review. Staff may need to consult program chairs, dissertation chairs, methodologists, and other experts as part of deciding how to, and who can, review a study and ensuring it meets the program outcomes and high standards for quality that Franklin University expects. A study may need to go to an IRB member or to the convened IRB for review. A study may present challenges or concerns to the IRB that require deep consideration and discussion. A study may include a research design with several moving parts that require strategizing. It really depends on the specific details of a study. Regardless of these details, IRB staff make every effort to conduct the pre-review and follow-up with the investigators as expeditiously as possible.
IRB staff reopen most studies with questions and/or comments for the investigator to address or consider before the IRB conducts a formal review and sends a determination letter to the study team. This is a normal part of the review process and does not mean the researcher has done anything wrong. We see a lot of studies and have a strong sense of what will and will not work once data collection begins. We also make suggestions to make data collection more efficient. For example, if an investigator plans to recruit participants in a way that we believe will be unsuccessful, the IRB Office will say so and work toward a solution. Our goal is for all Franklin researchers to find success in their research endeavors. Feedback from the IRB is intended to make a project better.
The submit button is still at the bottom of the blue 'Sections' column on the left side of your screen, but it's not as obvious when resubmitting. You will need to scroll down to the bottom of the blue column to find the 'Complete Submission' button. Click on the button.

Confirm that you want to continue with completing your submission.

After submitting your application, you still need to certify it. When you see this screen after confirming your submission, click on the blue 'Certify' button, under 'Routing', on the right side of your screen:
If you need to reopen your submission for any reason, you have the option to 'Return' the submission prior to certification.
Click 'Confirm' to certify your submission.
If you are a doctoral candidate, your certified submission will route to your dissertation chair for review and certification. You can see in Cayuse when the submission has been certified by the dissertation chair.
If you want to make a change to your study that needs to be reported to the IRB, you should open and submit a 'Modification' to your protocol. Submitting a modification follows the same process as opening and completing an 'Initial' submission.
To submit a modification to your protocol:
- Log in to Cayuse.
- Your landing page will say 'My Tasks' but otherwise be empty. At the top of your screen, on the right, you'll see 'Products'. Click on products and select 'Human Ethics', which will take you to your dashboard.
- Once you’re at your dashboard, open your study and select the blue ‘+ New Submission’ option in the upper right corner of the page. Select ‘Modification’ to complete and submit proposed changes to your study.
Closing a study in Cayuse follows the same process as opening and completing an 'Initial' or 'Modification' submission.
To close your protocol:
- Log in to Cayuse.
- Your landing page will say 'My Tasks' but otherwise be empty. At the top of your screen, on the right, you'll see 'Products'. Click on products and select 'Human Ethics', which will take you to your dashboard.
- Once you’re at your dashboard, open your study and select the blue ‘+ New Submission’ option in the upper right corner of the page. Select ‘Closure’ to complete and submit your final report.
Closed or withdrawn studies live in the archives. Use this document to find your study.
Cayuse keeps records of all documents related to your IRB protocol. To find a copy of your IRB determination (approval) letter, log in to Cayuse and open your study. When you open your study, you will be on the 'Study Details' page. On this page, you will see 'Submissions'. Click on the 'Submissions' tab.
.jpg)
When you click on the 'Submissions' tab, you will see a page that lists all submissions you have completed for the study, such as a modification. Click on your 'Initial' submission.
.jpg.png)
On the initial submission page, go to the tabs at the bottom of your screen and select 'Letters', where you will find your determination (approval) letter. You can download and print the letter once you click on it.
.jpg)
If you received an error stating “We don’t recognize that username”, you have not been added to Cayuse.
NOTE: Doctoral students are added to Cayuse once they are enrolled in DISS 9100.
If you feel you should have a user account in Cayuse but cannot login, contact the IRB Office at irb@franklin.edu.
Cayuse does experience an occasional glitch or will make systematic updates that may impact usability. If you get an error message, wait a few minutes and try logging in again. If the problem persists, please contact the IRB Office first (and not Cayuse support) at irb@franklin.edu. IRB staff can resolve most issues or confirm if there is a broader issue affecting users.
- Degrees
- Microcredentials & Certificates
- Admissions
- Tuition & Financial Aid
- Transferring Credit
- The Franklin Experience
- About Us
- FranklinWORKS Marketplace
- Safety & Security
- Policy Information
- Your Privacy Settings
- Privacy Policy
- Terms of Use
- Careers At Franklin
- Sitemap
Franklin University
201 S Grant Ave.
Columbus, OH 43215
Local: (614) 797-4700
Toll Free: (877) 341-6300
admissions@franklin.edu
Copyright 2025 Franklin University
Franklin University is accredited by the Higher Learning Commission (hlcommission.org/800.621.7440) and authorized by the Ohio Department of Higher Education.
Franklin University is committed to being an inclusive community free from all forms of discrimination and harassment.